
Feeding the World
Starch is one of the most important plant products for man kind. The major sources of starch for man’s use are the cereals, but roots and tubers are also important. Annual starch production from cereals is approximately 2,050 million tonnes and from roots and tubers 679 million tonnes.
The performance and properties of starch can be altered through modifications. Starch Modification is the process of enriching the properties of native starch through physical, chemical, enzymatic and by oxidation or addition of charged substituent’s to the polysaccharide backbone or combination of these methods to meet the demands of population growth rate through industrial production.
Chemical Oxidization is widely used method in modification of starch. Several oxidizing agents have been applied to starch oxidation including Sodium hypochlorite, Bromine, Periodate, Permanganate, Ammonium persulfate, Hydrogen peroxide, Ambient oxygen, Ozone, Chromic acid, Nitrogen dioxide and Ultraviolet radiation
Chemically Oxidized Starch
Oxidation of starch is one of a chemical modification. Oxidized starch is produced by reacting starch with a specific amount of oxidizing agents under controlled temperature and pH. Among them hypochlorite oxidation is the most common method for the production of oxidized starch in an industrial scale, because it is very cheap. Hydrogen peroxide, is the other oxidizing agent, has been used in a commercial practice.
During the course of reaction, several reactions occur which lead to the introduction of carbonyl and carboxyl groups which degrade the starch molecules. Hence, this method is important and widely used, because oxidized starch exhibits low viscosity due to de-polymerization , improves stability, clarity, film forming and binding properties. However, Chemical oxidations have several disadvantages.
Disadvantages with hypochlorite oxidation
- In the alkaline slurry generated by the hypochlorite oxidation process, the oxidized starch yield is low because of the loss of small molecules produced by starch breakdown.
- Formation of Chemical by products.
- Chemical odour and taste to the final product.
- Furthermore, in the process, large amounts of waste water, containing a high concentration of salts, cause waste water disposal problematic and increasing the operation costs.
Starch Quality
Starch consumers prefer white colour. But the colour is the first casualty in case of any imperfection or any shortcut deliberate or accidental in the manufacturing process. Some manufacturers resort to the ill-advised practice of adding chemicals like
- Bleaching agents (such as calcium hypochlorite, sodium hypochlorite,etc.,).
- Acids (such as hydrochloric acid, sulphuric acid & phosphoric acid, etc).
- Artificial & Optical whitening agents (such as 2-B-Con or Tinopal) are added to impart an artificial brilliant white colour to their improperly manufactured starch.
The chemically treated starch overcomes the handicap of colour but it is not good for the health of the consumer and attracts the Food Adulteration Act.
Ozone – An Organic and Green Technology for Natural Oxidation
What is Ozone?
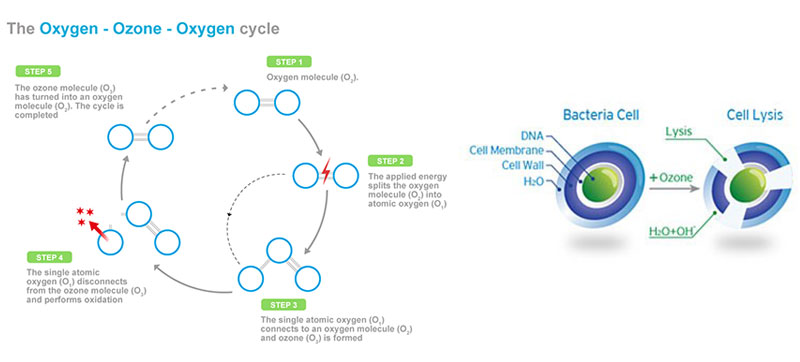
Ozone gas is a powerful form of oxygen and is also known as 03 or enriched oxygen or is tri atomic Oxygen That is, it contains three atoms of the oxygen molecule.
Because it is made up entirely of oxygen, ozone is sometimes called “activated oxygen”.
Because ozone is a strong naturally occurring oxidizing agent, it has been widely used in many industries for microbiological control, decolourization and organic compound decomposition. Ozone also occurs commonly in nature as a result of lightning strikes during thunderstorms. It is naturally occurring in small quantities. But generated as required in industrial scale, from the air surrounding us.
The “fresh, clean, spring rain” smell that we notice after a storm results from natures creation of ozone.
The three atom form of oxygen is a naturally occurring compound in the earth’s atmosphere. The life cycle of ozone is: generation, oxidation, and return to oxygen.
The air around us has 21% oxygen (O2). This is concentrated to 90% + with oxygen concentrators built in to the Ozone generator (Ozonator). This concentrated oxygen is then passed through an electrode inside the Ozonator, using corona discharge process, creates Ozone molecules (O3) from the oxygen molecules (O2).
How It Works:
- As ozone comes into contact with bacteria, the ozone molecule attacks the cell wall of the organism and breaks it down in a process known as cell lysing.
- Ozone is highly effective in oxidizing and destroying odours, viruses, molds and bacteria. It is effective in killing micro-organisms through oxidation of their cell membranes.
- Ozone has a unique property of auto-decomposition and will leave no toxic residues.
- It has an oxidation potential 1.5 times stronger than that of chlorine and has been shown to be effective over a much wider spectrum of microorganisms than chlorine and other disinfectants.
Advantages using Ozone for natural oxidation:
- Ozone kills bacteria such as Escherichia coli, Listeria, and other food pathogens much faster than traditionally used disinfectants, such as chlorine, and is free of chemical residues.
- Eliminate chlorine from a process: No THM’s or other chlorinated by-products.
- Implementing ozone reduces the risk of cross-contamination of pathogens.
- An ozone system mitigates the need for storage, handling, use, and disposal of chemical sanitation agents
- Ozone leaves no chemical residue: No Final Rinse – Less Water Usage
- Ozone improves taste and appearance over the use of chlorination alone: Better Quality Produce.
- The treated wash water is free of bacteria, colour, and suspended solids and can be recycled to reduce water usage. Ozone keeps wash water cleaner longer, there is Less Water Usage.
- An ozone treatment is capable of destroying pesticides and chemical residues in wash water and on cassava, so it is Chemical Free.
- In some situations, ozone reduces contamination in discharge water: Lower Cost Waste Water Disposal
- Gaseous ozone is a strong sanitation and fumigation agent and can be used to sanitize foods in the storage room and during shipping to prevent bacteria, mold, and yeast on the food surface and to control insects. Ozone lowers counts of spoilage microorganisms in wash water and on produce surface: Longer Shelf Life
- It can eliminate undesirable flavor produced by bacteria and chemically remove ethylene gas to slow down the ripening process, thus allowing extended distribution.
- For decades, it has been known that ozone is an effective disinfectant and sanitizer for the treatment of food products. It is commonly used in Europe for treatment of public water systems and food processing.
- Numerous documents and studies confirm the benefits of ozone applications in the food industry. Thus, ozone can successfully replace traditional sanitizing agents to control food pathogens.
The US FDA has approved the use of Ozone on raw agricultural commodities (RACs) in the preparing, packing, or holding of such commodities for commercial purposes. FDA-e.pdf
Applications in the Produce Industry
Many applications in the produce industry appear suitable for the use of ozone:
Process Water Sterilization.
Over the past several years, there has been increasing evidence that process water used by the food industry is not as free of pathogens as previously thought. Moreover, there is a certain level of pesticide and toxic organic compounds in the process water supply due to industrial activities.
Normally, processing water is disinfected and sterilized using chlorine. However, chlorine cannot reduce the level of organic compounds and will produce chlorinated compounds. Ozone has been proven to be an ideal replacement for chlorine for disinfection and sterilization of process water
According to the Environmental Protection Agency, ozone is the most effective primary disinfectant available for drinking water.
In fact, it is more effective than chlorine against microorganisms, including chlorine-resistant Cryptosporidium and Giardia which have invaded the food and water supplies and caused deaths in recent years.
The Ct value for 99% inactivation of Cryptosporidium is less than 2 mg min/L for ozone and higher than 30 for chlorine.
Ozone can also destroy chlorine byproducts, pesticides, and toxic organic compounds in the process water without any toxic residues. Practical applications of ozone to process water range from 0.5 to 5 ppm (depending on the water source), with less than 5 min contact time.
Fruit and Vegetable Storage.
Ozone is employed in cold storage of produce to guard against mold and bacteria at a very low concentration. It can destroy mold and bacteria in the air and on the surface of produce and also de-odourize.
Many early studies used gaseous ozone to prevent microbial activity on food surfaces and extend the shelf life of fruits and vegetables.
One of the important effects of ozone in cold storage is to slow down the fruit and vegetable ripening process. During ripening, many fruits, such as bananas and apples, release ethylene gas, which speeds up the ripening process. Ozone is very effective in removing ethylene through chemical reaction to extend the storage life of many fruits and vegetables.
Process Water Recycling.
Billion gallons of fresh water are used by the fresh produce industry annually. A need exists to decrease the quantity of water used, because of dramatically rising water and waste water treatment costs.
Ozone is a perfect candidate for treatment of water for recycling, since it is a powerful oxidizing agent that has been used to disinfect, to remove color, odor, and turbidity, and to reduce the organic loads of wastewater.
The use of ozone for waste water disinfection has been growing in popularity due to strict regulations on fecal coliform and other pathogens.
Ozone will destroy all bacteria without a preference to one type of organism.
Ozone will also remove some BOD, COD, and many other contaminates in the waste water stream.
Ozone helps in improved coagulation and turbidity removal.
Ozone oxidizes the transition metals like Arsenic (in presence of Iron), Cadmium, Chromium, Cobalt, Copper, Lead, Manganese, Nickel and Zinc to their higher oxidation state in which they usually form less soluble oxides, easy to separate by filtration. Ozone can be effectively used in the destruction of Cyanide.
Other Benefits:
Also, Ozone-treated starches, in general, exhibited an improved whiteness and paste clarity, lower paste viscosity and higher gel hardness.
The carbonyl and carboxyl groups increased significantly. While the amylose and amylopectin tended to be depolymerized as indicated by High Performance Size Exclusion chromatograms.
No alteration of starch granule morphology and crystallinity was evident. Changes in the molecular structure and physico-chemical and pasting properties of ozone-treated starches were likely corresponding to the properties of chemically oxidized starch, but having different degree of modification.
The degree of starch modification by ozone was influenced by ozone concentration and pH of starch slurry. When the ozone concentration and pH increased, a greater reduction of peak viscosity was observed.
Reference: Modification of cassava starch by ozonation, Researcher(s) Sitthichok Wanlapatit. Institution Cassava and Starch Technology Research Unit Source of Funds National Center for Genetic Engineering and Biotechnology National Science & Technology Development Agency
Use in Sugar mills
Because Ozone is a natural and effective oxidant, it is increasingly used in the sugar mills to replace chlorine products and other chemicals for refining and manufacture of pure sugar crystals with excellent white colour. This is approved by European and US FDA standards.
References:
http://www.google.com/patents/WO2003050313A3?cl=en
In conclusion, ozone can be used to produce naturally oxidized starch and the advantage that ozone has over other oxidizing agents is that it rapidly decomposes to di-atomic oxygen (O2) and does not leave harmful residues in final products.
